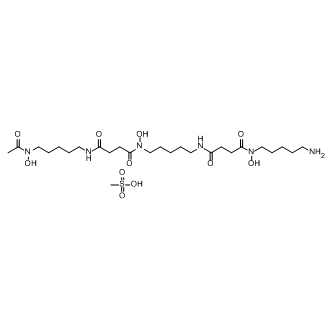
Deferoxamine mesylate
CAS No. 138-14-7
Deferoxamine mesylate( Desferrioxamine B mesylate | DFOM )
Catalog No. M11580 CAS No. 138-14-7
An iron chelator that binds iron and aluminium; increases HIF-1α transactivation in diabetes by preventing iron-catalyzed reactive oxygen stress.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | 45 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameDeferoxamine mesylate
-
NoteResearch use only, not for human use.
-
Brief DescriptionAn iron chelator that binds iron and aluminium; increases HIF-1α transactivation in diabetes by preventing iron-catalyzed reactive oxygen stress.
-
DescriptionAn iron chelator that binds iron and aluminium; increases HIF-1α transactivation in diabetes by preventing iron-catalyzed reactive oxygen stress.Other Indication Approved(In Vitro):Deferoxamine mesylate (1 mM; 16 h or 4 weeks) improves HIF-1α function under hypoxic and hyperglycemic conditions and decreases ROS in MEFs cells.Deferoxamine mesylate (100 μM; 24 h) increases InsR expression and activity and also induces an increase in p-Akt/total Akt/PKB levels.Deferoxamine mesylate (5, 10, 25, 50, 100 μM; 7 or 9 days) inhibits the proliferation of tumor-associated MSCs and bone marrow MSCs.Deferoxamine mesylate (5, 10, 25, 50, 100 μM; 7 days) induces apoptosis of MSCs.Deferoxamine mesylate (10 μM ; 3 days) influencs the expression of adhesion proteins on MSCs.Deferoxamine mesylate (100 μM; 24 h) induces autophagy mediated by the level of HIF-1α in SH-SY5Y cells.(In Vivo):Deferoxamine mesylate (560.68 mg/per; drip-on; once daily for 21 days) enhances wound healing and increases neovascularization in aged or diabetic mice.Deferoxamine mesylate (200 mg/kg; i.p.; daily for 2 weeks) results in HIF-1α stabilization and increases glucose uptake, hepatic InsR expression, and signaling in vivo.
-
In VitroWestern Blot AnalysisCell Line:MEFs cellsConcentration:1 mMIncubation Time:16 h (hypoxia condition); 4 weeks (hyperglycemic conditions)Result:Significantly attenuated the hyperglycemia-associated increase in ROS levels under hypoxic high glucose conditions.Notably increased normoxic HIF transactivation in MEFs under both high glucose and normal glucose conditions.Western Blot AnalysisCell Line:HepG2 cells Concentration:100 μM Incubation Time:24 hResult:Showed a twofold increase of InsR mRNA levels in cells.Increased by twofold InsR binding activity at the half-maximal concentration of 1.1 nM.Cell Proliferation Assay Cell Line:TAMSCs and BMMSCs (all isolated from Male C57BL/6J mice (8 week-old; EG-7 induced tumor model))Concentration:5, 10, 25, 50, 100 μMIncubation Time:7 days (TAMSCs); 9 days (BMMSCs)Result:Inhibited the growth of TAMSCs and BMMSCs, and most cells are died at day 7 or 9 when exposed to 50 and 100 μM dose.Apoptosis Analysis Cell Line:TAMSCs, BMMSCs Concentration:5, 10, 25, 50, 100 μM Incubation Time:7 days Result:Exhibited proapoptotic effect on TAMSCs and BMMSCs cells.Western Blot AnalysisCell Line:TAMSCs, BMMSCs Concentration:10 μM Incubation Time:3 days Result:Remarkably decreased VCAM-1 expression in both TAMSCs and BMMSCs.Cell Autophagy AssayCell Line:SH-SY5Y cells Concentration:100 μM Incubation Time:24 h Result:Increased the ratio of LC3-II/I, an indicator of autophagy, which effects were blocked when autophagy-related gene Beclin 1 was suppressed by Beclin 1 siRNA transfection.
-
In VivoAnimal Model:Aged (21-month-old) and diabetic (12-week-old) C57BL/6J mice (excisional wound model).Dosage:6.57 μg/per (10 uL of 1 mM)Administration:Drip-on; once daily for 21 days.Result:Displayed significantly accelerated healing and increased neovascularization in both aged and diabetic mice model.Animal Model:Male Sprague-Dawley rats (180-200 g).Dosage:200 mg/kg Administration:Intraperitoneal injection; daily for 2 weeks.Result:Significantly increased hepatic HIF-1α protein levels, InsR protein levels, as well as Akt/PKB and activated Akt/PKB were significantly higher in the liver.
-
SynonymsDesferrioxamine B mesylate | DFOM
-
PathwayOthers
-
TargetOther Targets
-
RecptorAluminum|Iron
-
Research AreaOther Indications
-
IndicationOther Disease
Chemical Information
-
CAS Number138-14-7
-
Formula Weight656.7897
-
Molecular FormulaC26H52N6O11S
-
Purity>98% (HPLC)
-
SolubilityH2O: ≥ 33 mg/mL
-
SMILESCS(O)(=O)=O.CC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCN
-
Chemical NameButanediamide, N4-[5-[[4-[[5-(acetylhydroxyamino)pentyl]amino]-1,4-dioxobutyl]hydroxyamino]pentyl]-N1-(5-aminopentyl)-N1-hydroxy-, methanesulfonate (1:1)
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Duscher D, et al. Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):94-9.
2. Miyajima H, et al. Ann Neurol. 1997 Mar;41(3):404-7.
3. Choi EY, et al. J Immunol. 2004 Jun 1;172(11):7069-77.
molnova catalog



related products
-
Tangeretin
Tangeretin, a flavonoid from citrus fruit peels, has been proven to play an important role in anti-inflammatory responses and neuroprotective effects in several disease models, and was also selected as a Notch-1 inhibitor.
-
ND-011992
ND-011992 is a reversible and selective quinazoline inhibitor targeting quinone reductases and quinol oxidases.ND-011992 inhibits E. coli BL21*Δcyo respiratory complex I.
-
N-Benzylmethylamine
N-Benzylmethylamine is an Alkaloids.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


